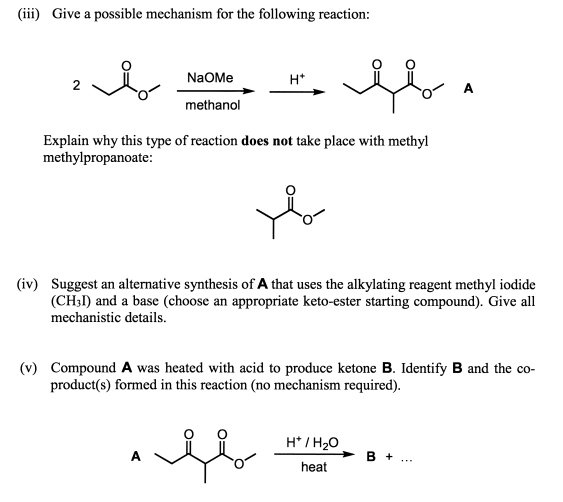
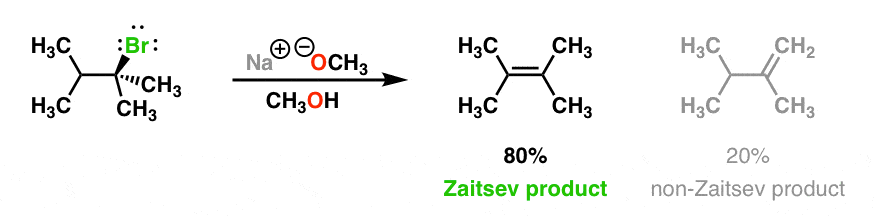
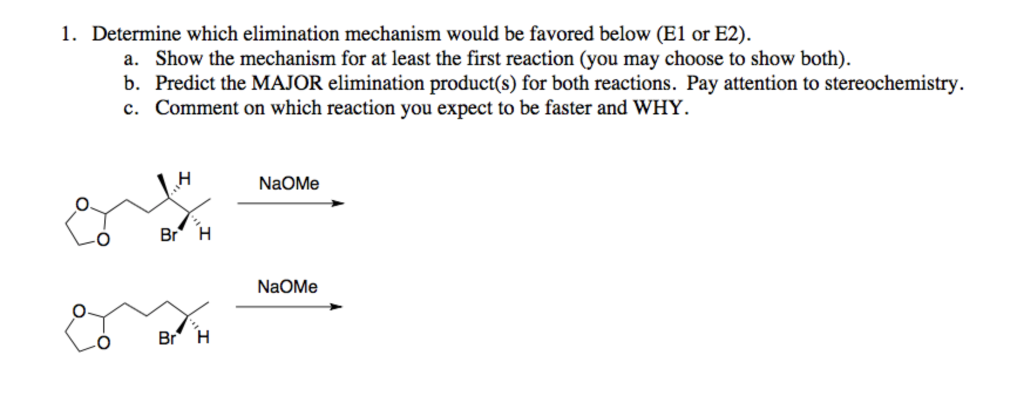
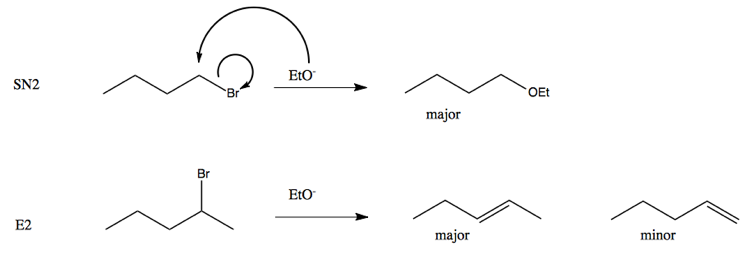
Supply structures for H through K. Given : " An Aldohexose "overset (MH2OH"/ base ")(to)H overset (Ae2O"/"NaOAC)(to)I overset (-HOAC)(to)J overset (NaOMe "/"MeOH)(to)K.

Which of the following would be the best base for performing the following elimination? A. NaOH B. NaOMe C. KOtBu D. H2O | Homework.Study.com
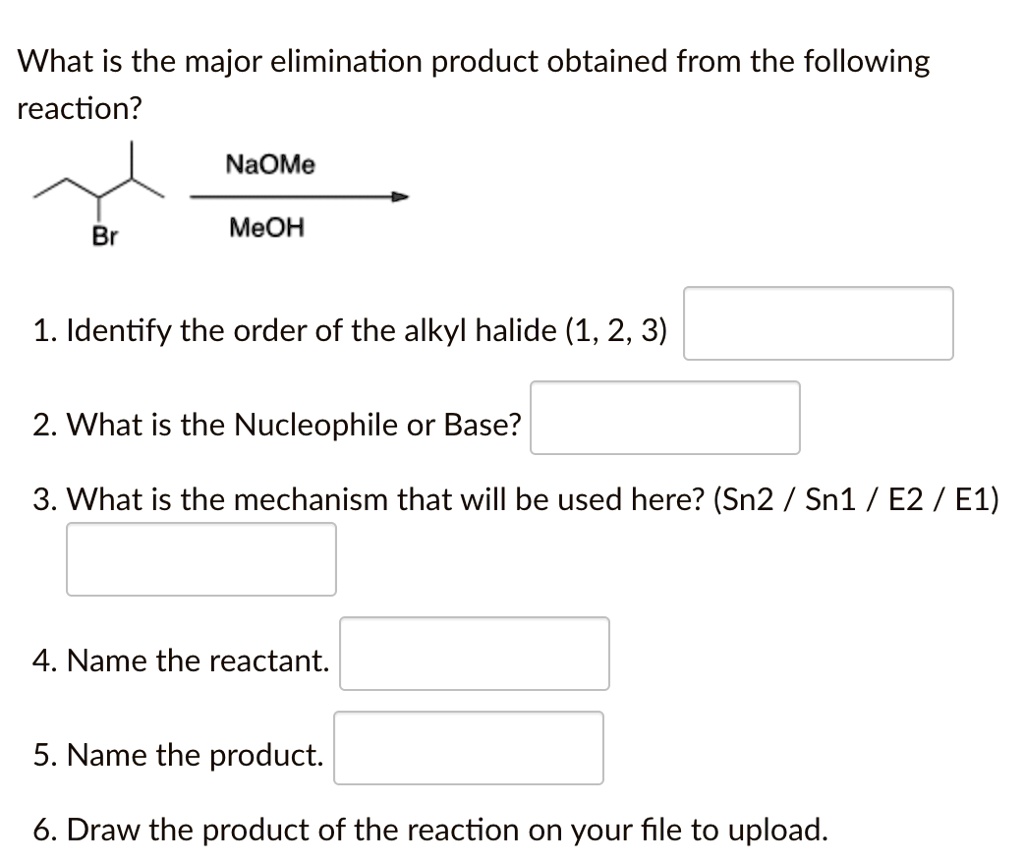
SOLVED: What is the major elimination product obtained from the following reaction? NaOMe Br MeOH 1. Identify the order of the alkyl halide (1,2,3) 2. What is the Nucleophile or Base? 3.

if we had used NaOMe instead of T-BuOK,wouldn't we have ended with the same product? since we have only on Beta postion? : r/chemhelp

Scheme 2. Nucleophilic addition of NaOMe onto 1: acetal formation under... | Download Scientific Diagram